 Image from vaginalmeshlawsuit.com
Image from vaginalmeshlawsuit.com In October 2008, the Food and Drug Administration (FDA) released their first safety notification regarding the use of a transvaginal mesh in repair of pelvic organ prolapse (POP) and stress urinary incontinence (SUI). As stated in this notification, the bureau has received more than 1,000 complaints regarding the use of a vaginal mesh. However, despite the number of complaints, the FDA concluded that the complications arising from the use of a vaginal mesh were rare.
In July 2011, the FDA was able to release their second update on the safety and effectiveness of a vaginal mesh during POP and SUI surgeries. According to the second report showing a significant increase of complaints as compared to the first report, the complications associated with a vaginal mesh surgery were not rare. From the 2,874 complaints filed to the regulatory board of the FDA, 1,503 complaints were related to a pelvic organ prolapse repair whilst 1,371 complaints were in relation to a stress urinary incontinence procedure. It is because of this significant increase in numbers that the FDA has released a safety guide on the proper use of a vaginal mesh during surgery.
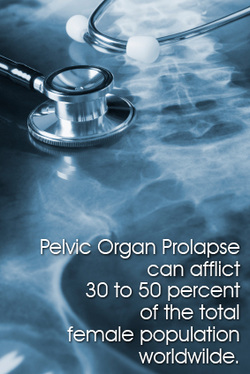
A vaginal mesh is made from synthetic materials, which are most often non-absorbable, materials. It may come in different sizes or shapes and they are likely to have ‘wings’. These wings, better known by doctors as trocar, are the junctures which will holds the mesh in place once it has been anchored into the vaginal area. Since POP and SUI conditions are often caused by the lowering of the pelvic organs into the vagina, the mesh will act as a suspension, holding the organs up in their proper places.
Complications that are most usually associated with a vaginal mesh implant differ per disorder. In a pelvic organ prolapse, the most common complications are vaginal erosion, pelvic pains, bladder perforation, blood vessel perforation and pain during copulation or dyspareunia. In a SUI mesh implant, complications such as bleeding, infections and recurrent incontinence issues may prevail.
The FDA has stated that multiple complications might arise during post-operation. With the number of complaints that the FDA has been receiving and with the number of patients out there seeking vaginal mesh lawsuit compensation, the regulatory board highly recommends doctors and health care workers to fully-inform their patients about the potential complications and risks involved in a transvaginal mesh implant.
REFERENCES:
fda.gov/medicaldevices/safety/alertsandnotices/publichealthnotifications/ucm061976.htm
fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/UroGynSurgicalMesh/default.htm
In July 2011, the FDA was able to release their second update on the safety and effectiveness of a vaginal mesh during POP and SUI surgeries. According to the second report showing a significant increase of complaints as compared to the first report, the complications associated with a vaginal mesh surgery were not rare. From the 2,874 complaints filed to the regulatory board of the FDA, 1,503 complaints were related to a pelvic organ prolapse repair whilst 1,371 complaints were in relation to a stress urinary incontinence procedure. It is because of this significant increase in numbers that the FDA has released a safety guide on the proper use of a vaginal mesh during surgery.
A vaginal mesh is made from synthetic materials, which are most often non-absorbable, materials. It may come in different sizes or shapes and they are likely to have ‘wings’. These wings, better known by doctors as trocar, are the junctures which will holds the mesh in place once it has been anchored into the vaginal area. Since POP and SUI conditions are often caused by the lowering of the pelvic organs into the vagina, the mesh will act as a suspension, holding the organs up in their proper places.
Complications that are most usually associated with a vaginal mesh implant differ per disorder. In a pelvic organ prolapse, the most common complications are vaginal erosion, pelvic pains, bladder perforation, blood vessel perforation and pain during copulation or dyspareunia. In a SUI mesh implant, complications such as bleeding, infections and recurrent incontinence issues may prevail.
The FDA has stated that multiple complications might arise during post-operation. With the number of complaints that the FDA has been receiving and with the number of patients out there seeking vaginal mesh lawsuit compensation, the regulatory board highly recommends doctors and health care workers to fully-inform their patients about the potential complications and risks involved in a transvaginal mesh implant.
REFERENCES:
fda.gov/medicaldevices/safety/alertsandnotices/publichealthnotifications/ucm061976.htm
fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/UroGynSurgicalMesh/default.htm
 RSS Feed
RSS Feed